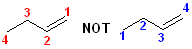
Alkenes
(unsaturated hydrocarbons have one or more carbon carbon double bond. They have the general formula: CnH2n and are also known by an older term: olefins)
Basic IUPAC nomenclature: (See the home page for parent root names, prefixes & suffixes. The following modifications are used for alkenes.)
1. Number the longest chain of carbon atoms that contains the double bond in the direction that gives the carbons of the double bond the lowest numbers. Although the two carbons define the location of the double bond, only the first carbon atom is numbered-by definition, the next carbon atom must be part of the alkene. So, the compound below has 4 carbons and is a but- and the ending -ene to form butene , but the position of the beginning of the double bond must be specified.
2. Locate the double bond by the number of its first carbon. In this compound, the double bond begins at carbon #1, so the full name becomes: 1-butene.
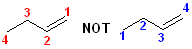
Note the INcorrect numbering in the second structure. There is no such compound
as 3-butene.
3. After the longest chain, containing the double bond is identified as the root name, number the carbons. Then, name the substituents. Assemble the name by alphabetizing the substitutents, with their numbers and combining with the parent name.
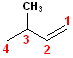 This
compound has a longest chain, containing the alkene, of 4 carbons. It is a
but- + -ene = butene. The double bond begins
on carbon #1, so it is specifically a 1-butene
(like the example above). However, it also has a methyl substitutent at the #3
carbon; the name will have to include 3-methyl. Thus, the correct name is:
3-methyl-1-butene.
This
compound has a longest chain, containing the alkene, of 4 carbons. It is a
but- + -ene = butene. The double bond begins
on carbon #1, so it is specifically a 1-butene
(like the example above). However, it also has a methyl substitutent at the #3
carbon; the name will have to include 3-methyl. Thus, the correct name is:
3-methyl-1-butene.
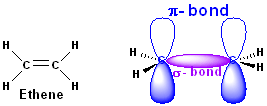
4. Cis-trans isomerism in alkenes. Alkenes contain a carbon-carbon double bond, and although the common representation of the structure uses two single lines (see below) the two components of the double bond are not the same. Part one, is called a sigma s- bond-it is formed from the overlap of sp2 hybrid orbitals; the second part, is called a p-bond and is formed by the overlap of two p orbitals. The two bonds "lock" the two carbon atoms together in such a way, that there is NO rotation around the double bond; it is "frozen" in place. In many alkenes, the structure is symmetric, and there is no issue of stereochemistry.
However, many alkenes can exist in two isomeric forms, yet have the same connectivity of atoms (i.e. constitutional isomers). These isomer differ in their orientation of groups, and are therefore, geometric isomers. Consider 2-butene (CH3CH=CHCH3):
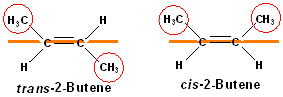 Both
compounds are named as 2-butenes, but two geometric
isomers are possible: using the double bond as a reference plane or
line-(see orange line), the two methyl groups are either on the same side of the
double bond (this is called cis) or the they may be on opposite sides
(this is called trans). Just as the case with cyclic alkanes, a name
might not specify the stereochemistry-in this case, 2-butene. But, the
stereochemistry CAN be included in the name by placing cis or trans
in front of the IUPAC name, separating it with a hyphen; it is usually
italicized.
Both
compounds are named as 2-butenes, but two geometric
isomers are possible: using the double bond as a reference plane or
line-(see orange line), the two methyl groups are either on the same side of the
double bond (this is called cis) or the they may be on opposite sides
(this is called trans). Just as the case with cyclic alkanes, a name
might not specify the stereochemistry-in this case, 2-butene. But, the
stereochemistry CAN be included in the name by placing cis or trans
in front of the IUPAC name, separating it with a hyphen; it is usually
italicized.
It is important to note, that hydrogens are NOT included in this type of nomenclature-only groups or atoms other than hydrogen are considered. In the example below, the geometric isomers refer to the orientation of the chlorine atoms, NOT the hydrogen atoms.
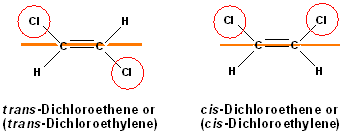
Notice, that a second name has been introduced with these structures. The base name is different: ethene has been replaced with "ethylene." This is an example of the use of common or nicknames in nomenclature and it is discussed below.
5. Common names in alkenes. some alkenes, particularly low-molecular-weight alkenes, are known almost exclusively by their common names:

EXERCISE: Write the IUPAC name for each of the following structures.
| Structure | Name | Structure | Name | ||
| 1 | 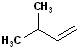 |
|
11 | 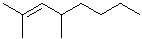 |
|
| 2 | 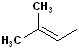 |
|
12 | 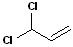 |
|
| 3 |  |
|
13 | 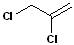 |
|
| 4 | 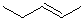 |
|
14 |  |
|
| 5 |  |
|
15 |  |
|
| 6 | 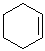 |
|
16 | 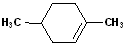 |
|
| 7 | 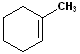 |
|
17 | 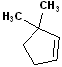 |
|
| 8 | 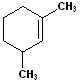 |
|
18 | 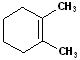 |
|
| 9 | 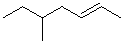 |
|
19 |  |
|
| 10 | 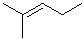 |
|
20 | 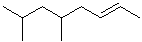 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret