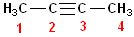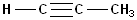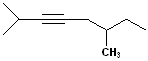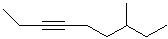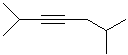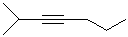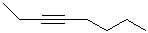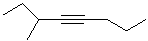Alkynes
(unsaturated hydrocarbons have one or more carbon carbon triple bond. They have the general
formula: CnH2n-2 )
Basic IUPAC nomenclature:
(See the home page for parent root names, prefixes & suffixes. The following
modifications are used for alkynes.)
1. Number the longest chain of carbon atoms that contains the triple bond in the
direction that gives the carbons of the triple bond the lowest numbers. Although
the two carbons define the location of the triple bond, only the first carbon
atom is numbered-by definition, the next carbon atom must be part of the alkyne.
For alkynes, use the infix -yn- and the parent name
of the alkane.
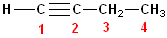 So, this compound has 4 carbons and is a butane
carbon chain, and inserting the infix -yn-
gives the name:
butyne , but the position of the beginning of the triple bond must be
specified. The triple bond begins on C1 and, as is the case with alkenes, it is
not necessary to use two numbers to specify its location, so the full, proper
name is: 1-butyne.
Because the triple bond is
located on the end of the carbon chain, these are sometimes referred to, as "terminal
alkynes."
So, this compound has 4 carbons and is a butane
carbon chain, and inserting the infix -yn-
gives the name:
butyne , but the position of the beginning of the triple bond must be
specified. The triple bond begins on C1 and, as is the case with alkenes, it is
not necessary to use two numbers to specify its location, so the full, proper
name is: 1-butyne.
Because the triple bond is
located on the end of the carbon chain, these are sometimes referred to, as "terminal
alkynes."
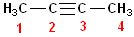 This
is an isomer of 1-butyne; it is named 2-butyne,
because the triple bond begins on C2. Notice, that the compound can be numbered
left to right or right to left and the name is still 2-butyne. Because the
triple bond is located on the "interior" of the carbon chain, these are
sometimes referred to, as "internal alkynes."
This
is an isomer of 1-butyne; it is named 2-butyne,
because the triple bond begins on C2. Notice, that the compound can be numbered
left to right or right to left and the name is still 2-butyne. Because the
triple bond is located on the "interior" of the carbon chain, these are
sometimes referred to, as "internal alkynes."
2.
For alkynes, which have other
substitutents, or branching of the carbon chain, the rules for alkenes apply:
number the longest chain containing the triple bond, number, name & alphabetize
the substitutents. Note: the triple bond has linear geometry, and as such, there
is no possibility of cis or trans isomerism.
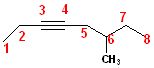 The
parent name of this compound is octane and the
triple bond begins on C3. So, the parent name of the compound is:
3-octyne. There is a methyl- group at C6, so the
full name becomes: 6-Methyl-3-octyne.
The
parent name of this compound is octane and the
triple bond begins on C3. So, the parent name of the compound is:
3-octyne. There is a methyl- group at C6, so the
full name becomes: 6-Methyl-3-octyne.
3. Common names in alkynes.
The smallest
member of this family (C2H2) is the two carbon unit:
ethyne. However, this is known, almost exclusively,
by its common name: acetylene. The common
nomenclature uses acetylene as the parent name and the substitutents on either
side are added, in front, as separate words. Using 2-butyne as the example, if
the common name, acetylene, were used, then each of the methyl groups
would represent the two sides of the structure, and no numbering would be
necessary. The common name would be: Dimethylacetylene.
The compound below could be named, Methylacetylene.
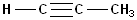
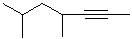
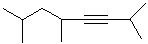
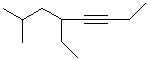
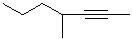
EXERCISE: Write the IUPAC name for each
of the following structures.
END OF SECTION
email questions & comments to:
Dr. JA Colapret
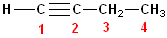 So, this compound has 4 carbons and is a butane
carbon chain, and inserting the infix -yn-
gives the name:
butyne , but the position of the beginning of the triple bond must be
specified. The triple bond begins on C1 and, as is the case with alkenes, it is
not necessary to use two numbers to specify its location, so the full, proper
name is: 1-butyne.
Because the triple bond is
located on the end of the carbon chain, these are sometimes referred to, as "terminal
alkynes."
So, this compound has 4 carbons and is a butane
carbon chain, and inserting the infix -yn-
gives the name:
butyne , but the position of the beginning of the triple bond must be
specified. The triple bond begins on C1 and, as is the case with alkenes, it is
not necessary to use two numbers to specify its location, so the full, proper
name is: 1-butyne.
Because the triple bond is
located on the end of the carbon chain, these are sometimes referred to, as "terminal
alkynes."