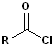
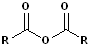
Derivatives of Carboxylic Acids
This section deals with 4 categories of compounds, which are related to carboxylic acids:
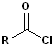 |
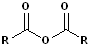 |
 |
 |
| Acid Chlorides | Acid Anhydride | Ester | Amide |
The common structural feature is the acyl group:
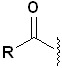 For
these functional groups, the carbonyl group was once part of a carboxylic acid,
and for that reason, they are considered derivatives of carboxylic acids. The
nomenclature of each of these will be discussed in turn and example problems are
grouped together at the end of the section.
For
these functional groups, the carbonyl group was once part of a carboxylic acid,
and for that reason, they are considered derivatives of carboxylic acids. The
nomenclature of each of these will be discussed in turn and example problems are
grouped together at the end of the section.
Acid Chlorides
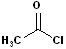
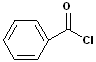
The acyl group bonded to a halogen is called an acyl halide; the most common are acid chlorides. The name is constructed by identifying the carboxylic acid and changing the suffix to -yl halide. Thus, propanoic acid will become propanoyl chloride. Common names, used for certain acids, are retained in the acid chlorides e.g. acetic acid --> acetyl chloride and benzoic acid--> benzoyl chloride. Diacids are likewise retained in the corresponding diacid chlorides e.g. oxalylic acid--> Oxalyl chloride.
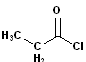 |
 |
 |
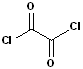 |
| Propanoyl Chloride | Acetyl Chloride | Benzoyl Chloride | Oxalyl Chloride |
Acid Anhydrides
Two acyl groups bonded to an common oxygen atom is a carboxylic anhydride. These names are easily constructed by replacing the acid with anhydride. Thus, acetic acid becomes acetic anhydride, benzoic acid, benzoic anhydride etc. There is also a large group of cyclic anhydrides, which are derived from intramolecular diacids:
|
|
|
|
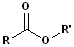
Carboxylic Esters
When the carboxyl group is bonded to an -OR or an -OAr, the functional group is called an carboxylic ester or just an ester. These may be viewed as having two parts: an acid component, which contains the carbonyl and an ether segment, which contains the -OR group:
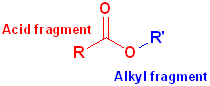 The
acid fragment forms the base name of the compound and the suffix
-ic acid is changed to -ate.
The ether fragment will contain an alkyl or an aryl group and it is named in the
normal fashion, and placed, in front of the base name.
The
acid fragment forms the base name of the compound and the suffix
-ic acid is changed to -ate.
The ether fragment will contain an alkyl or an aryl group and it is named in the
normal fashion, and placed, in front of the base name.
| Ester Structure | Acid Name | Ether | Ester |
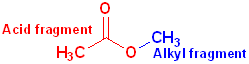 |
Acetic Acid | Methyl | Methyl acetate |
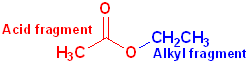 |
Acetic Acid | Ethyl | Ethyl acetate |
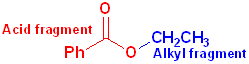 |
Benzoic Acid | Ethyl | Ethyl Benzoate |
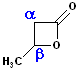
Cyclic Esters
Esters, which are arranged in a cycle are called: lactones. Name the parent carboxylic acid, drop the suffix -ic acid and add -olactone.
 |
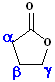 |
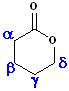 |
| 4 C acid: butyric | 4 C acid: butyric | 5 C acid: pentanoic |
| 3-Butanolactone | 4-Butanolactone | pentanolactone |
| b-butyrolactone | g-butyrolactone | d-pentanolactone |
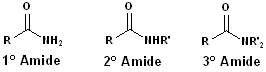
Amides
When the carboxyl group is bonded to a nitrogen atom, the functional group is called an amide. IUPAC: the -oic acid is dropped from the parent compound and -amide is added. E.G. benzoic acid becomes benzamide.
Common name: drop the -ic from the parent name & add -amide. E.G. acetic becomes acetamide.
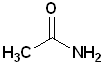
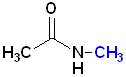
Since, the nitrogen atom can be bonded to the carbonyl and two other atoms, amides can be classified as 1o, 2o or 3o. The location of such groups are specified by the use of N- as a prefix. Each group on the nitrogen atom is specified:
 |
 |
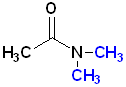 |
| Acetamide | N-Methylacetamide | N,N-Dimethylacetamide |
| 1o amide | 2o amide | 3o amide |
Cyclic Amides
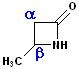
Amides, which are arranged in a cycle are called: lactams. Name the parent carboxylic acid, drop the suffix -ic acid and add -lactam.
 |
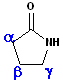 |
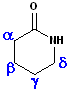 |
| 4 C acid: butyric | 4 C acid: butyric | 5 C acid: pentanoic |
| 3-Butanolactam | 4-Butanolactam | pentanolactam |
| b-butyrolactam | g-butyrolactam | d-pentanolactam |
EXERCISE: Write the IUPAC name for each of the following structures.
| Structure | Name | Structure | Name | |||
| 1 | 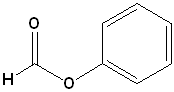 |
|
6 |
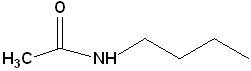 |
|
|
| 2 | 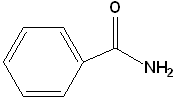 |
|
7 |
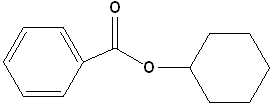 |
|
|
| 3 | 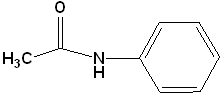 |
|
8 |
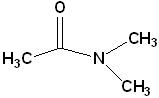 |
|
|
| 4 | 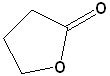 |
|
9 |
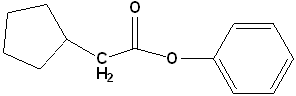 |
|
|
| 5 | 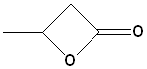 |
|
10 |
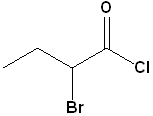 |
|
|
END OF SECTION
email questions & comments to: Dr. JA Colapret