Stereochemistry
The study of spatial (3-D) arrangement of atoms within molecules.
Basic Definitions:
1) Isomers: different compounds with the same molecular formula.
2) Constitutional isomers: Isomers with different connectivity of atoms. (How the atoms are attached to each other)
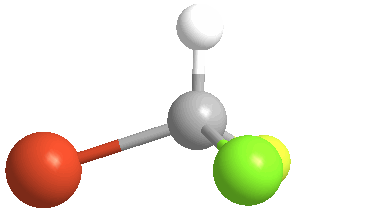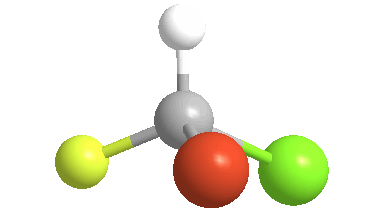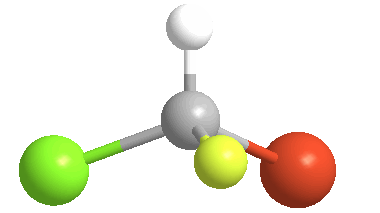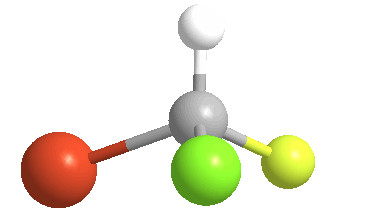
3) Stereoisomers: compounds with the same connectivity, but different orientations in 3-D space. As an example, examine the orientation of each of the atoms in bromochlorofluoromethane:

(color coding for the atoms: white = hydrogen; yellow = fluorine; green = chlorine & orange = bromine)
4) Chiral (Gr kheir-"hand"): This is a property of objects (e.g. molecules), which are asymmetric, that is, the object possess NO geometric element of symmetry, such as a plane, axis, or center. These objects are nonsuperposable upon their mirror image. Consider bromochlorofluoromethane, here shown with its mirror image:
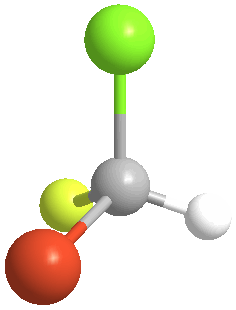
|
 |
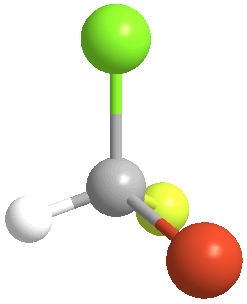 |
There is no way to rotate, turn or orient the two models to superpose upon each other:
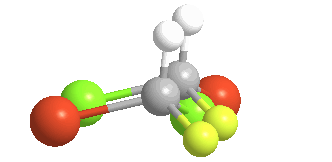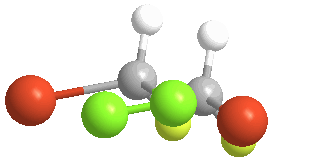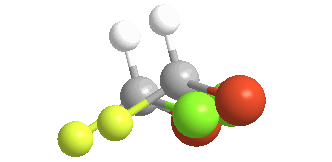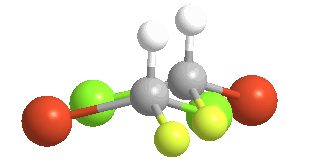
Of the 5 atoms, 3 can be "put on each other" (superposable), but the other two will never match up. In this example, the green atoms (Chlorine) and the orange (Bromine) atoms do not match; the other 3 atoms do. The molecule is said to be "chiral" and the carbon is called a Chiral center. An especially helpful way to recognize chiral carbons is to look for 4 different groups (or atoms) attached to the central carbon:
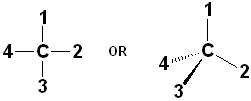 These
are but two ways to draw a generic chiral carbon. The drawing on the left, of
course, does not indicate any stereochemistry at all- it is a flat drawing, but
the central C atom is chiral.
However, the drawing with a dashed line & wedge, does depict stereochemistry:
the C and groups # 1 & 2 are in the plane of the paper; group #3 comes out of
the plane (towards the viewer) and group #4 goes back, away from the viewer.
These
are but two ways to draw a generic chiral carbon. The drawing on the left, of
course, does not indicate any stereochemistry at all- it is a flat drawing, but
the central C atom is chiral.
However, the drawing with a dashed line & wedge, does depict stereochemistry:
the C and groups # 1 & 2 are in the plane of the paper; group #3 comes out of
the plane (towards the viewer) and group #4 goes back, away from the viewer.
5) Enantiomers: These are pairs of nonsuperposable, mirror images. Bromochlorofluoromethane is NOT superposable upon its mirror image, and therefore, the two stereoisomers are an enantiomeric pair or enantiomers of each other.
EXERCISE: Indicate which Carbon atoms (if any) are chiral in the following structures.
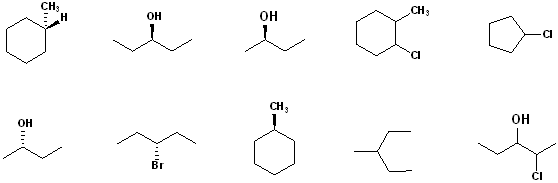
Using the R & S designations for Chiral Compounds
Priority Rules:
1) The four different groups (or atoms) attached to a chiral carbon are assigned a priority, based on the atomic number:
| Atomic # --> | 1 | 6 (the C atom) | 7 (the N atom) | 8 (the O atom) | 16 (the S atom) | 17 | 35 |
| -H | -CH3 | -NH2 | -OH | -SH | -Cl | -Br | |
| ---Increasing Priority----> | |||||||
2) If priority cannot be assigned per the atoms bonded to the chiral center, look to the next set of atoms; priority is assigned at the first point of difference:
| Atomic # --> | 1 | 6 (the C atom) | 7 (the N atom) | 8 (the O atom) | 16 (the S atom) | 17 | 35 |
| --CH2-H | --CH2-CH3 | --CH2-NH2 | --CH2-OH | --CH2-SH | --CH2-Cl | --CH2-Br | |
| ---Increasing Priority----> | |||||||
The atom, which marks the "first point of difference" is highlighted in pink. Notice that the priority of the groups is analogous to rule #1.
3) Atoms that are part of a double or triple bond are considered to be bonded to an equivalent number of similar atoms by single bonds:
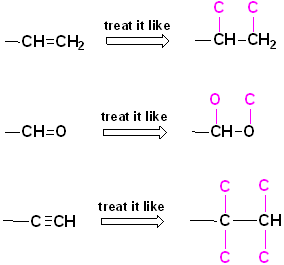
Assigning R & S designations for Chiral Centers
1. Locate the chiral center and identify its four substituents. Remember to look for 4 different groups or atoms attached to a central carbon atom.
2. Assign priority from 1 (highest) to 4 (lowest) to each substituent. Use the priority rules above; if two atoms are the same, then keep going along the structure till there is a difference. (Note: if there is NO difference, then that carbon is NOT chiral.)
3. Orient the molecule so that the group of lowest priority (4) is directed away from you. Again, using bromochlorofluoromethane, each of the two stereoisomers (the pair of enantiomers) will give these two perspectives:
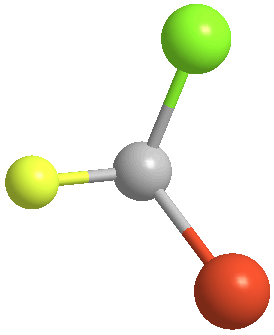 |
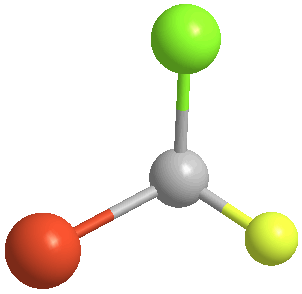 |
4. Label the groups, using the priority rules, from 1-3. If the numbering is clockwise, then it is defined as the R configuration (R latin-rectus: right); the counterclockwise direction is defined as S (S latin-sinister: left). Here is the process, used on each enantiomer of bromochlorofluoromethane:
|
|
Rotate to the right-hide the hydrogen, and that will look like this--------> |
 |
Counter-Clockwise rotation-to the "left"- S-configuration |
|
|
Rotate to the left-hide the hydrogen, and that will look like this--------> |
 |
Clockwise rotation-to the "right"- R-configuration |
R & S designations are part of the nomenclature of chiral compounds. The designation is placed in the front of the name, in parenthesis and usually in italics. It is separated from the rest of the name by a hyphen. Now, the full, IUPAC names for the two enantiomers are:
|
(S)-bromochlorofluoromethane |
(R)-bromochlorofluoromethane |
EXERCISE: Assign R or S configurations to the following compounds and provide the full IUPAC name for each. (It is helpful to build models of the compounds.)
| Structure | Name | Structure | Name | ||
| 1 | 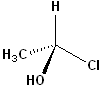 |
|
11 | 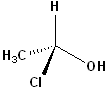 |
|
| 2 | 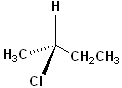 |
|
12 |  |
|
| 3 | 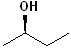 |
|
13 | 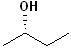 |
|
| 4 |  |
|
14 |  |
|
| 5 | 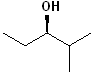 |
|
15 | 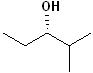 |
|
| 6 | 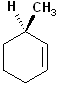 |
|
16 | 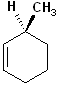 |
|
| 7 | 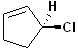 |
|
17 | 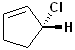 |
|
| 8 | 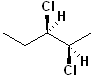 |
|
18 | 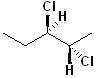 |
|
| 9 | 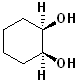 |
|
19 | 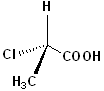 |
|
| 10 | 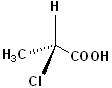 |
|
20 | 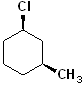 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret