Carboxylic Acids
(The functional group of this class is the carboxyl group: -COOH or -CO2H)
Basic IUPAC nomenclature-CARBOXYLIC ACIDS: (See the home page for parent root names, prefixes & suffixes.)
1. In the IUPAC system, the -e is dropped from the parent alkane, and the suffix -oic acid is added. For example, the 4 C chain of butane will become: butan- + -oic acid = butanoic acid. The C atom of the COOH group will become the origin for numbering- it will be C1. Since the carboxyl group will be at the end of a structure, the use of an actual number in the name is not necessary. (e.g. it is understood that butanoic acid will have C1 as the carboxyl group). Although a formal IUPAC name exits for each compound, many of the carboxylic acids have common names which are used almost exclusively. Some of these are shown in Table 1.
Table 1. The common & IUPAC names for the first 10 carboxylic acids
| Carbon atoms | Common name | IUPAC name | Chemical formula |
|---|---|---|---|
| 1 | Formic acid | Methanoic acid | HCOOH |
| 2 | Acetic acid | Ethanoic acid | CH3COOH |
| 3 | Propionic acid | Propanoic acid | CH3CH2COOH |
| 4 | Butyric acid | Butanoic acid | CH3(CH2)2COOH |
| 5 | Valeric acid | Pentanoic acid | CH3(CH2)3COOH |
| 6 | Caproic acid | Hexanoic acid | CH3(CH2)4COOH |
| 7 | Enanthic acid | Heptanoic acid | CH3(CH2)5COOH |
| 8 | Caprylic acid | Octanoic acid | CH3(CH2)6COOH |
| 9 | Pelargonic acid | Nonanoic acid | CH3(CH2)7COOH |
| 10 | Capric acid | Decanoic acid | CH3(CH2)8COOH |
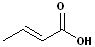
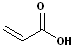
2. If the compound contains a double bond i.e. it is also an alkene, then change the infix from -an- to -en- and the placement of the infix is determined by the numbering.
 |
 |
| trans-2-Butenoic acid | Propenoic acid |
| (Crotonic Acid) | (Acrylic Acid) |
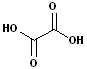
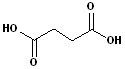
3. There are an abundance of compound which have two carboxyl functional groups and these are known as: dicarboxylic acids. To construct the name of these compounds, add the suffix -dioic acid to the name of the parent alkane containing both carboxyl groups. Like their counterparts, many of these also have a common names.
 |
 |
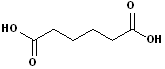 |
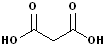 |
| Ethanedioic Acid | Butanedioic Acid | Hexanedioic Acid | Propanedioic Acid |
| (Oxalic Acid) | (Succinic Acid) | (Adipic Acid) | (Malonic Acid) |
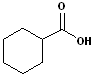
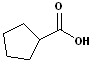
4. When the carboxyl functional group is attached to a ring, the entire suffix:-carboxylic acid is used. Examples are:
 |
 |
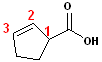 |
| Cyclohexane carboxylic acid | Cyclopentane carboxylic acid | 2-Cyclopentene carboxylic acid |
| The ring system takes precedence for the base name: cyclopentene; the carboxyl group is attached to carbon #1. |
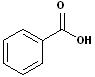
5. Aromatic acids: When aromatic compounds contain the carboxyl group, attached to the ring, the compound is considered an Aromatic acid. The most common example is benzoic acid. While many derivatives may use benzoic acid as the base name, here too, there are many common names and nicknames. There are also examples of aromatic diacids. There is much more to the nomenclature of aromatic compounds, that may be found on the aromatic page of this site. Some examples include:
 |
 |
 |
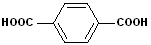 |
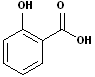
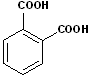
| Benzoic Acid | 2-Hydroxybenzoic acid | 1,2-Benzenedicarboxylic acid | 1,4-Benzenedicarboxylic acid |
| (Salicylic Acid) | Phthalic acid | Terephthalic acid |
EXERCISE: Write the IUPAC name for each of the following structures.
| Structure | Name | Structure | Name | ||
| 1 |  |
|
6 |  |
|
| 2 | 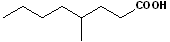 |
|
7 | 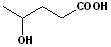 |
|
| 3 | 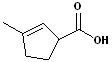 |
|
8 |  |
|
| 4 | 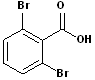 |
|
9 | 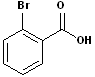 |
|
| 5 | 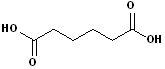 |
|
10 | 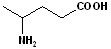 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret