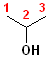
Alcohols
(The classes of compounds, in which a carbon atom is connected to a hydroxyl group)
Basic IUPAC nomenclature-Alcohols (See the home page for parent root names, prefixes & suffixes.)
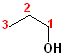
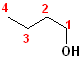
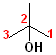
1) The longest continuous chain, containing the -OH group will be the parent chain.
2) Number the parent chain, such that the carbon bearing the -OH group has the lowest number.
3) Change the -e of the alkane to -ol.
The first few members of this family would be named: methane ---> methanol; ethane---> ethanol; propane--->propanol and butane---> butanol.
Common Names
All alcohols will also have a common name, which is often used interchangeably with the IUPAC name. The common name is formatted by identifying the alkyl group attached to the O atom and adding, as a separate word, the term alcohol. Methanol is also known as methyl alcohol; ethanol is ethyl alcohol, etc.
Some examples of the two nomenclature styles are shown in the Table:
 |
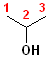 |
 |
 |
|
| IUPAC | 1-Propanol | 2-Propanol | 1-butanol | 2-methyl-2-propanol |
| COMMON | n-Propyl alcohol | isopropyl alcohol | n-butyl alcohol | t-butyl alcohol |
Unsaturated Alcohols
1) For those compounds which contain both a double bond and a hydroxyl group, the infix -an- is changed to -en- and the suffix -ol is added.
2) Number the carbon chain, containing both the -OH and the alkene to give the hydroxyl the lowest carbon number.
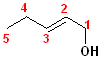
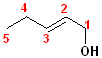 This
compound is a pentene and also an alcohol. The carbons are numbered so that C1
contains the hydroxyl group; the double bond is between C2 & C3, so it is a
2-ene. thus, it becomes 2-penten +
1-ol = 2-penten-1-ol.
This compound can also exist as geometric isomers. To address that aspect of the
nomenclature, E is incorporated into the full name. The proper formatted name
becomes: (E)-2-penten-1-ol.
This
compound is a pentene and also an alcohol. The carbons are numbered so that C1
contains the hydroxyl group; the double bond is between C2 & C3, so it is a
2-ene. thus, it becomes 2-penten +
1-ol = 2-penten-1-ol.
This compound can also exist as geometric isomers. To address that aspect of the
nomenclature, E is incorporated into the full name. The proper formatted name
becomes: (E)-2-penten-1-ol.
Multiple Hydroxyl Groups
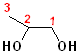
Many compounds have more than one hydroxyl group; two hydroxyl groups are called "diols". If the hydroxyl groups are on adjacent carbons, then it is called a "glycol." If the hydroxyl groups are on the same carbon, then it is called a "gem" (for geminal, or twin) diol. The parent name of the alkane is incorporated, and the term "diol" is added. Thus, the ethane derivative, containing two hydroxyl groups, becomes ethane + diol and adding the numbers of the hydroxyl groups: 1,2-Ethanediol. The common name of such compounds uses the term "glycol" as part of the name. The first term of such names is the alkene, from which the diol was derived (by hydroxylation).
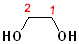 |
 |
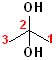 |
| 1,2-Ethanediol | 1,2-Propanediol | 2,2-Propanediol |
| Ethylene glycol | Propylene glycol | (a gem-diol) |
Classification of Alcohols
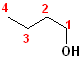
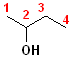
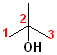
In addition to the IUPAC nomenclature system, it is common practice to classify alcohols according to the carbon to which the hydroxyl group is attached. Classifying functional groups in this fashion is especially helpful when studying the variety of reactions of a particular group. For example, primary and secondary alcohols can be oxidized, but tertiary alcohols cannot. Some examples are shown in the table:
 |
 |
 |
 |
|
| IUPAC | 1-butanol | (E)-2-Penten-1-ol | 2-Butanol | 2-methyl-2-propanol |
| Common | n-butyl alcohol | (E)-2-Pentenyl-1-acohol | 2-Butyl alcohol | t-butyl alcohol |
| Classification | Primary (1o) | Primary (1o) [also allylic] | Secondary (2o) | Tertiary (3o) |
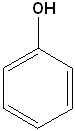
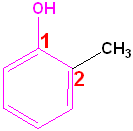
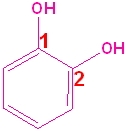
Aromatic Alcohols
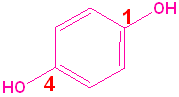
When a hydroxyl group is attached directly to a benzene ring, the compound is called a "phenol." The parent compound is phenol and the carbon attached to the OH group is C1. In derivatives of phenol, the other functional group(s) are alphabetized & numbered. Alternatively, disubstituted penols, may use the ortho-, para and meta- nomenclature (see Benzene & Derivatives). The table below shows some representative examples.
 |
 |
 |
 |
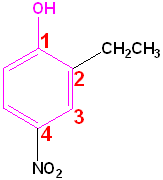 |
| Phenol | 2-Methylphenol | 1,2-Benzenediol | 1,4-Benzenediol | 2-Ethyl-4-nitrophenol |
| (Hydroxybenzene) | (o-Cresol) | (Catechol) | (Hydroquinone) | (no common name) |
EXERCISE: Write the IUPAC name for each of the following structures.
| STRUCTURE | NAME | STRUCTURE | NAME | ||
| 1 | 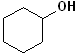 |
|
11 | 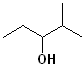 |
|
| 2 | 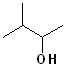 |
|
12 | 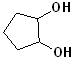 |
|
| 3 | 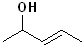 |
|
13 | 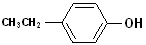 |
|
| 4 | 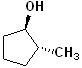 |
|
14 | 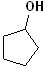 |
|
| 5 |  |
|
15 | 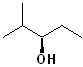 |
|
| 6 | 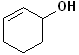 |
|
16 | 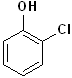 |
|
| 7 | 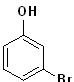 |
|
17 |  |
|
| 8 | 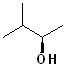 |
|
18 | 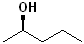 |
|
| 9 |  |
|
19 | 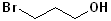 |
|
| 10 | 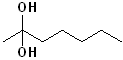 |
|
20 | 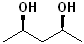 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret