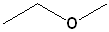 The
longest chain is the ethane; the OR group is
methoxy. Thus, the name becomes
Methoxy- + ethane =
Methoxyethane.
The
longest chain is the ethane; the OR group is
methoxy. Thus, the name becomes
Methoxy- + ethane =
Methoxyethane. Ethers & Epoxides
(The classes of compounds, in which two carbon atoms are connected to an oxygen atom; epoxides are the 3-membered, cyclic version. )
Basic IUPAC nomenclature-Ethers (See the home page for parent root names, prefixes & suffixes.)
1) The longest continuous carbon chain will be the parent name.
2) Name the -OR group as a substituent.
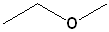 The
longest chain is the ethane; the OR group is
methoxy. Thus, the name becomes
Methoxy- + ethane =
Methoxyethane.
The
longest chain is the ethane; the OR group is
methoxy. Thus, the name becomes
Methoxy- + ethane =
Methoxyethane.
3) If there are two alkoxy groups, they are handled in the same fashion. They would be diethers.
 The
longest chain is still ethane, but now, there are two alkoxy substituents, and
they are both methoxy groups. Numbers are used to describe the positions of the
alkoxy groups. Thus, this name becomes:
1,2-Dimethoxyethane also known by its abbreviation: DME.
The
longest chain is still ethane, but now, there are two alkoxy substituents, and
they are both methoxy groups. Numbers are used to describe the positions of the
alkoxy groups. Thus, this name becomes:
1,2-Dimethoxyethane also known by its abbreviation: DME.
Common Names
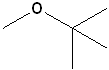
All ethers will also have a common name, which is often used interchangeably with the IUPAC name. To assemble the common name, the two groups flanking the O atom are named, alphabetized and the word "ether" is added.
Some examples:
 |
 |
 |
 |
|
| IUPAC | Ethoxyethane | 1-Methoxy-1,1-dimethylethane | Methoxyethane | 1-Methoxybutane |
| Common | Diethyl ether | Methyl-t-Butyl ether (MTBE) | Ethyl methyl ether | Butyl methyl ether |
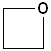
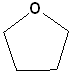
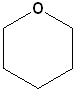
Basic nomenclature-Cyclic Ethers
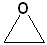
Cyclic ethers have IUPAC names and the prefix ox- indicates the presence of oxygen in the ring system. Suffixes indicate the number of atoms in the ring. The first few members of this series are shown below:
 |
 |
 |
 |
|
| IUPAC | Oxirane | Oxetane | Oxolane | Oxane |
| Common | ethylene oxide | none | tetrahydrofuran | tetrahydropyran |
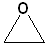
Basic nomenclature-Epoxides
1) Simple epoxides are named as derivatives of oxirane.
2) Where the epoxide is part of another ring system, it is shown by the prefix epoxy-.
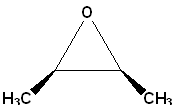
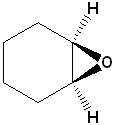
3) Common names are derived from the name of the alkene from which the epoxide is formally derived; name the alkene and the term oxide is added.
 |
 |
 |
|
| IUPAC | Oxirane | cis-2,3-Dimethyloxirane | 1,2-Epoxycyclohexane |
| Common | ethylene oxide | cis-2-butene oxide | cyclohexene oxide |
EXERCISE: Write the IUPAC name for each of the following structures.
| STRUCTURE | NAME | STRUCTURE | NAME | ||
| 1 | 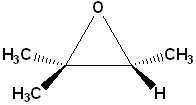 |
|
6 | 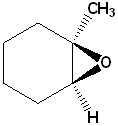 |
|
| 2 | 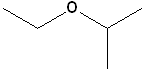 |
|
7 | 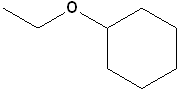 |
|
| 3 | 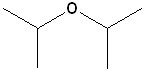 |
|
8 | 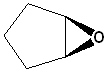 |
|
| 4 | 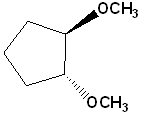 |
|
9 | 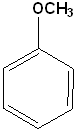 |
|
| 5 |  |
|
10 | 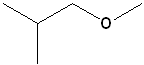 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret