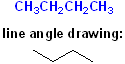
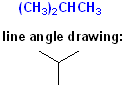
Chapter 2 of the textbook has the initial discussion of nomenclature. In each subsequent chapter, the nomenclature of each class of compounds is discussed, with examples for variation of the nomenclature rules. This site contains explanations & various exercises for each class of organic compounds. Both the systematic IUPAC and the common names (where appropriate) are illustrated. Use these exercises as a workbook-that is, write down each of your answers before checking the answer. Part of reinforcing the rules is to actually write the name of the compound. Check the spelling & punctuation carefully, since only a single letter difference can indicate a different class of compounds. (E.G. alkanes vs alkenes)
The exercises are graduated from easy (basic rules) to more complex structures & names. To navigate directly to another class (functional group) of compounds, follow the links below.
| Alkenes | Alcohols | Carboxylic Acids | Amines |
| Alkynes | Ethers & Epoxides | Derivatives of Carboxylic Acids | |
| Haloalkanes | Aldehydes & Ketones | Benzene & Its Derivatives |
Access a special section for stereochemistry here.
Alkanes (saturated hydrocarbons arranged in an open chain. They have the general formula: CnH(2n+2)
Constitutional isomers: Compounds with the same molecular formula but a different connectivity of their atoms.
For example, there are two isomers of butane; both have the formula of C4H10 but the Carbon atoms are connected in a different fashion:
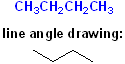
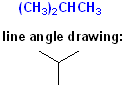
The linear (straight line) structure is the "normal" isomer and is named: n-butane. The second drawing shows the carbon skeleton with a bend, more commonly called a "branch" (like the branch of a tree) and is given the common name: isobutane.
Parent or root name: The longest continuous C chain is used to determine the root (or parent) name of the compound. An effective technique to identify the longest chain, is to draw a line connecting the maximum # of Cs, without lifting the pen from the paper. The root name is identified by the # of carbons in the longest chain. Note: there will often be more C atoms in the molecule, but only those in the continuous chain are used for the root name. Use the chart below for the parent name:
| Prefix | C # | Prefix | C # | |
| meth- | 1 | hex- | 6 | |
| eth- | 2 | hept- | 7 | |
| prop- | 3 | oct- | 8 | |
| but- | 4 | non- | 9 | |
| pent- | 5 | dec- | 10 |
Notice, the table only has the prefix portion of the name. The rest of the name will identify the class of organic compounds. In this section, the class of alkanes is the subject and the ending (rest of the name) will be -ane. For example, the 1 carbon alkane will be named: meth- plus the ending -ane = methane.
EXERCISE: Write out the formal names for each of the hydrocarbons having these formulas.
| C# | Name | C# | Name |
| 2 |
|
7 |
|
| 3 |
|
8 |
|
| 4 |
|
9 |
|
| 5 |
|
10 |
|
| 6 |
|
1 |
|
Substituent: A group of atoms, which are connected to the longest continuous C chain. These may be hydrocarbon groups or may contain other atoms, such as oxygen, nitrogen etc. For the hydrocarbon groups, they are called Alkyl groups and derive their name from the number of C atoms in that group. To generalize and simplify the use of an organic group, the symbol "R" is frequently used. To name the groups, simply add a -yl to the prefixes; for example, the one carbon group becomes: meth- + -yl = methyl.
EXERCISE: Write out the names of each of these alkyl groups.
| C# | Name | C# | Name |
| 2 |
|
7 |
|
| 3 |
|
8 |
|
| 4 |
|
9 |
|
| 5 |
|
10 |
|
| 6 |
|
1 |
|
THE IUPAC SYSTEM (International Union of Pure & Applied Chemistry)
Every compound will have a systematic name, which can be assembled using the nomenclature rules of the IUPAC. In addition, thre will be many compounds which will also have a common or nickname. The two systems are used frequently, but it is important to understand that every compound will have an IUPAC name, NOT every compound will have a common or nickname.
1.The name of a saturated hydrocarbon with an unbranched chain consists of a prefix and suffix.
2. The parent chain is the longest chain of carbon atoms.
3. Each substituent is given a name and a number. Use a hyphen to connect the number to the name. Use a comma to separate numbers from each other.
For the isomers of butane:
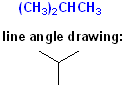 The
nickname or common name is isobutane, but the IUPAC name is 2-methylpropane.
The
nickname or common name is isobutane, but the IUPAC name is 2-methylpropane.
Note, that there are 4 carbon atoms, but the compound is named as a derivative of propane.
To arrive at that name, the longest continuous chain is identified. It will be a 3 C chain:
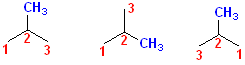 Shown
here are three different ways to number a 3 carbon chain; the methyl group is
shown, in each case to be connected to the #2 carbon. Regardless of which
drawing is used, the methyl group is still on the #2 carbon. So, the name is
assembled by: identify the parent chain, propane;
number the chain, any of the choices shown. Number & name the substitutent: it
is a methyl group on the #2 position, so that part
becomes, 2-methyl. Now, put the fragments together:
2-Methylpropane. It is written as a single word;
the number goes in front of the group occupying that position. The methyl group
is on the 2 position; that is formatted as shown not:
Shown
here are three different ways to number a 3 carbon chain; the methyl group is
shown, in each case to be connected to the #2 carbon. Regardless of which
drawing is used, the methyl group is still on the #2 carbon. So, the name is
assembled by: identify the parent chain, propane;
number the chain, any of the choices shown. Number & name the substitutent: it
is a methyl group on the #2 position, so that part
becomes, 2-methyl. Now, put the fragments together:
2-Methylpropane. It is written as a single word;
the number goes in front of the group occupying that position. The methyl group
is on the 2 position; that is formatted as shown not:
Methyl-2-propane.
4. If there is one substituent, number the chain from the end that gives it the lower number.
 Notice
that the numbering begins on the right, in this particular drawing; however, the
same compound can be drawn differently, but the Cs are still numbered such that
the substitutent (a methyl group) is on the #2 Carbon.
Notice
that the numbering begins on the right, in this particular drawing; however, the
same compound can be drawn differently, but the Cs are still numbered such that
the substitutent (a methyl group) is on the #2 Carbon.
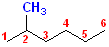 Both of
these drawings represent the same compound. The name is assembled by identifying
the parent chain, which is a 6 carbon chain, thus
it is a hexane. The substitutent is a
methyl group and it is on the # 2 position. Thus,
the name becomes: 2-methyl + hexane =
2-Methylhexane.
Both of
these drawings represent the same compound. The name is assembled by identifying
the parent chain, which is a 6 carbon chain, thus
it is a hexane. The substitutent is a
methyl group and it is on the # 2 position. Thus,
the name becomes: 2-methyl + hexane =
2-Methylhexane.
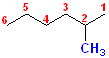
This is the same structure with the wrong numbering:
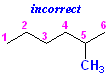 In fact,
there is no such compound as "
In fact,
there is no such compound as "5-Methylhexane." That name is
invalid.
5. If there are two or more identical substituents, number the chain from the end that gives the lower number to the substituent encountered first. Indicate the number of times the substituent appears by a prefix di-, tri-, tetra-, and so on. Use commas to separate position numbers.
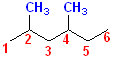 Numbering
the longest C chain gives a hexane, with two methyl groups @ C# 2 and 4. The
name is assembled by identifying the parent chain, which is a 6 carbon chain, thus
it is a hexane. There are 2 methyl groups, so that
part of the name becomes: dimethyl. To indicate
where the two methyl groups are located, use the C# of the parent chain,
numbering the chain, so that the lowest numbers are used. In this case, C # 2
and 4 mark the position of the two methyl groups. Now, the dimethyl part of the
name can be specified: 2,4-Dimethyl. Thus, the name
becomes: 2,4-Dimethyl + hexane =
2,4-Dimethylhexane.
Numbering
the longest C chain gives a hexane, with two methyl groups @ C# 2 and 4. The
name is assembled by identifying the parent chain, which is a 6 carbon chain, thus
it is a hexane. There are 2 methyl groups, so that
part of the name becomes: dimethyl. To indicate
where the two methyl groups are located, use the C# of the parent chain,
numbering the chain, so that the lowest numbers are used. In this case, C # 2
and 4 mark the position of the two methyl groups. Now, the dimethyl part of the
name can be specified: 2,4-Dimethyl. Thus, the name
becomes: 2,4-Dimethyl + hexane =
2,4-Dimethylhexane.
This is the same structure with the wrong numbering:
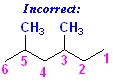 In fact,
there is no such compound as "
In fact,
there is no such compound as "3,5-Dimethylhexane." That name is
invalid.
6. If there are two or more different substituents, list them in alphabetical order, number from the end of the chain that gives the substituent encountered first the lower number.
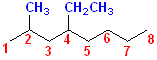 Numbering
the longest C chain gives an octane, with a methyl group @ C# 2 and an ethyl
group @ 4. With this rule, alphabetize the parts first, then assign the lowest
numbers. The parent name is octane. The
ethyl group will come before the
methyl group, so it will become: ethyl + methyl +
octane. Now, to specify where the methyl & ethyl groups are located, but retain
alphabetical order: 4-Ethyl + 2-methyl + octane =
4-Ethyl-2-methyloctane.
Numbering
the longest C chain gives an octane, with a methyl group @ C# 2 and an ethyl
group @ 4. With this rule, alphabetize the parts first, then assign the lowest
numbers. The parent name is octane. The
ethyl group will come before the
methyl group, so it will become: ethyl + methyl +
octane. Now, to specify where the methyl & ethyl groups are located, but retain
alphabetical order: 4-Ethyl + 2-methyl + octane =
4-Ethyl-2-methyloctane.
The most common mistake applying this rule, is to
number the substitutents before alphabetizing or to transpose the parts, i.e.
2-Methyl-4-ethyloctane.
7. The prefixes di-, tri-, tetra-, (and so on) are not included in alphabetization.
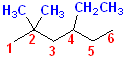 Numbering
the longest C chain gives a hexane, with two methyl groups (thus, dimethyl) and
one ethyl group. The name is assembled: ethyl + dimethyl + hexane. However, the
ethyl will appear before the methyl groups, even though there are two methyl
groups and they will become DImethyl. In other words, alphabetize the groups,
ignore how many there are. Ethyl will appear before the methyl. The numbering
will follow, to give the lowest numbers to all the groups. Now, the name can be
assembled: 4-Ethyl + 2,2-Dimethyl + hexane =
4-Ethyl-2,2-dimethylhexane. Notice that the #2 must be used twice. Since
there are 2 methyl groups, the position of each must be specified. In this case,
both are on the same C, namely, #2.
Numbering
the longest C chain gives a hexane, with two methyl groups (thus, dimethyl) and
one ethyl group. The name is assembled: ethyl + dimethyl + hexane. However, the
ethyl will appear before the methyl groups, even though there are two methyl
groups and they will become DImethyl. In other words, alphabetize the groups,
ignore how many there are. Ethyl will appear before the methyl. The numbering
will follow, to give the lowest numbers to all the groups. Now, the name can be
assembled: 4-Ethyl + 2,2-Dimethyl + hexane =
4-Ethyl-2,2-dimethylhexane. Notice that the #2 must be used twice. Since
there are 2 methyl groups, the position of each must be specified. In this case,
both are on the same C, namely, #2.
Common Names for Branched Alkanes
Some of the branches of alkanes are used so often, that they have their own common names. These are listed below:
| NAME | Condensed Structural Formula | Line Angle Formula | Comments |
| Isopropyl | 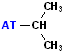 AT--CH(CH3)2 |
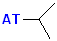 |
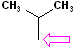 Attachment is @ the stem of the "Y" |
| Isobutyl | 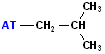 AT--CH2CH(CH3)2 |
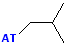 |
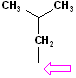 Attachment is @ the stem of the "Y" |
| sec-butyl or s-butyl | 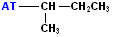 AT--CH(CH3)(CH2CH3) |
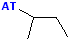 |
Attachment is @ one of the secondary positions |
| tert-butyl or t-butyl | 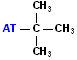 AT--C(CH3)3 |
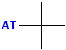 |
Look for the "T" or cross; the attachment is at the base of the "t". |
| iso-pentyl | 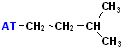 AT--CH2CH2CH(CH3)2 |
 |
Notice the "Y" & the attachment to the base of the Y-it's just 2 C atoms away. |
| neo-pentyl | 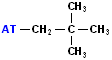 AT--CH2C(CH3)3 |
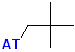 |
The term "neo" means new & it refers to a new type of C. Namely-a quaternary C (the C in the middle). |
NOTE: "AT" represent the connection site for the rest of the molecule. It may be an individual atom, such as a Cl or a group of atoms such as OH (hydroxide).
EXERCISE: Write out the names for each of these compounds.
|
STRUCTURE FORMULA |
COMMON NAME | IUPAC NAME |

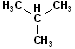 |
|
|
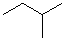
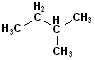 |
|
|
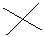
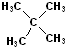 |
|
|
Using Nomenclature Segments (IUPAC)
Prefix: Tells the number of carbon atoms in the parent chain. (See chart above; e.g. eth- for 2 carbons)
Infix: Tells the nature of the carbon-carbon bonds in the parent chain.
|
INFIX |
Class of Compounds |
Example | Comments |
| --an-- | Alkanes | Propane | C3H8: No unsaturation |
| --en-- | Alkenes (1 or more double bonds) | Propene | C3H6: 1 double bond |
| --yn-- | Alkynes (1 or more triple bonds) | Propyne | C3H4: 1 triple bond |
Suffix: Tells the class of the compound.
|
SUFFIX |
Class of Compounds |
Example |
| --e | Hydrocarbons | Propane |
| --ol | Alcohol | Propanol |
| --al | Aldehyde | Propanal |
| --amine | Amine (base) | Propanamine |
| --one | Ketone | Propanone |
| --oic acid | Carboxylic Acid | Propanoic acid |
EXERCISE: Identify the segments for each compound; then assemble the IUPAC name.
| STRUCTURE | PREFIX | INFIX | SUFFIX | IUPAC NAME |
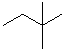 |
But- | --an-- | -e |
|
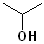 |
Pro- | --an-- | -ol |
|
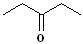
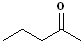 |
Pent- | --an-- | -one |
|
 |
Pent- | --an-- | -one |
|
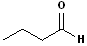 |
But- | --an-- | -al |
|
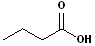 |
But- | --an-- | -oic acid |
|
 |
Hex- | --an-- | -amine |
|
 |
Hex- | --an-- | -ol |
|
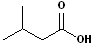 |
But- | --an-- | -oic acid |
|
 |
But- | --an-- | -ol |
|
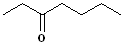 |
Hep- | --an-- | -one |
|
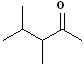 |
Pent- | --an-- | -one |
|
 |
But- | --en-- | -e |
|
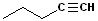 |
Pen- | --yn-- | -e |
|
Cyclic Alkanes (IUPAC)
Cyclic alkanes are compounds in which the carbon atoms form a circle. They have the general formula: CnH2n , but are still considered saturated aliphatic hydrocarbons. They are easily named by using the prefix cyclo with the appropriate fragment. The smallest member of the family contains 3 carbons and is shaped as a triangle. Since it is a prop- (3C) and still and alkane, the root name is propane; however, it is a cyclic (circle) molecule, so its name becomes cyclopropane. Cyclic compounds, up to 8 carbons are listed below. They are all alkanes.

The basic rules for IUPAC:
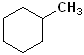 Consider this compound. The 6 C, arranged in a circle defines
the compound as a cyclo- and
hexane = cyclohexane.
There is a methyl group attached to the ring, and that will add the term
"methyl" to the root name. Now, the full name becomes:
Methyl + cyclohexane =
Methylcyclohexane. It is NOT necessary to use a number, since there is
only one substitutent; it does not matter how the structure is drawn or its
orientation; it is still Methylcyclohexane.
Consider this compound. The 6 C, arranged in a circle defines
the compound as a cyclo- and
hexane = cyclohexane.
There is a methyl group attached to the ring, and that will add the term
"methyl" to the root name. Now, the full name becomes:
Methyl + cyclohexane =
Methylcyclohexane. It is NOT necessary to use a number, since there is
only one substitutent; it does not matter how the structure is drawn or its
orientation; it is still Methylcyclohexane.
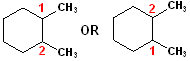 When
two substitutents are present, each must be named and their positions specified.
The root name of the compound is cyclohexane and that accounts for those 6 C
atoms; as the base name, those 6 C atoms have priority over any other groups
attached to the ring. In other words, the numbering goes around the ring, NOT
outside of it-even though that might result in a longer chain of C atoms: the
ring is the parent name. In this case, cyclohexane. There are two Methyl groups,
so Dimethyl will be used; furthermore, the methyl groups are on positions # 1 &
2, regardless of numbering clockwise or counterclockwise. Notice, that the
numbering pattern gives the lowest numbers: 1 & 2.
When
two substitutents are present, each must be named and their positions specified.
The root name of the compound is cyclohexane and that accounts for those 6 C
atoms; as the base name, those 6 C atoms have priority over any other groups
attached to the ring. In other words, the numbering goes around the ring, NOT
outside of it-even though that might result in a longer chain of C atoms: the
ring is the parent name. In this case, cyclohexane. There are two Methyl groups,
so Dimethyl will be used; furthermore, the methyl groups are on positions # 1 &
2, regardless of numbering clockwise or counterclockwise. Notice, that the
numbering pattern gives the lowest numbers: 1 & 2.
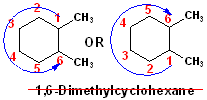 Here is
the same compound with the wrong numbering. The
base name is still cyclohexane and there are still two methyl groups, thus,
Dimethylcyclohexane. However, this numbering has one of the C atoms wrong: #6
instead of a lower #2.
Here is
the same compound with the wrong numbering. The
base name is still cyclohexane and there are still two methyl groups, thus,
Dimethylcyclohexane. However, this numbering has one of the C atoms wrong: #6
instead of a lower #2.
In fact,
there is no such compound as "1,6-Dimethylcyclohexane." That name is
invalid.
Stereochemistry in Cyclic Alkanes (IUPAC)
One of the more interesting and important features of organic structures, is their arrangement of the atoms in 3 D space, or stereochemistry. In order to understand the way this depicted in two dimensional drawings, two drawing forms are introduced here, namely, wedges and dashed lines. They look like this:
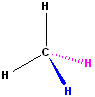 This is
the simple methane molecule with 3D characteristics drawn into the structure.
The plane of the paper defines a plane of reference; bonds can come out of the
plane towards the reader or out of the plane, away from the reader. The
wedge bond is intended to "come out of the page"
towards you. The dashed bond is intended to go "an equal distance, away" from
you.
This is
the simple methane molecule with 3D characteristics drawn into the structure.
The plane of the paper defines a plane of reference; bonds can come out of the
plane towards the reader or out of the plane, away from the reader. The
wedge bond is intended to "come out of the page"
towards you. The dashed bond is intended to go "an equal distance, away" from
you.
There are then, 3 atoms remaining in the plane of the
paper-namely, the H--C--H (shown in black).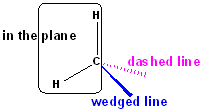
Here is the line angle drawing of octane and the same structure drawn with 3D dashes and wedges for some of the H atoms:
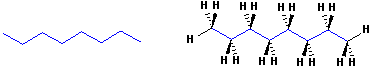 Normally,
the use of dashes & wedges is limited to carbons atoms, in which the
stereochemistry is important. With simple compounds like this one, the dashes &
wedges give a "3D" perspective, but there are no stereochemical differences in
the drawings.
Normally,
the use of dashes & wedges is limited to carbons atoms, in which the
stereochemistry is important. With simple compounds like this one, the dashes &
wedges give a "3D" perspective, but there are no stereochemical differences in
the drawings.
The cyclic alkanes, however, can have differences in stereochemistry. This occurs when there are more than one substitutent on the ring-there is a relationship between the groups, and that can be represented, using the dashes & wedges.
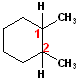 Consider
this structure: it is a six C ring, therefore it is a cyclohexane- (that is the
parent name of the compound); there are two methyl groups attached to the ring,
so, that will add a Dimethyl term to the name and finally, the methyl groups are
specifically attached to Carbons # 1 & 2 (regardless of numbering clockwise or
counterclockwise). Assembling the name: 1,2-Dimethyl
+ cyclohexane = 1,2-Dimethylcyclohexane. There is no
stereochemistry indicated in this drawing, but the compound can have differences
in the relationship of the two methyl groups to each other.
Consider
this structure: it is a six C ring, therefore it is a cyclohexane- (that is the
parent name of the compound); there are two methyl groups attached to the ring,
so, that will add a Dimethyl term to the name and finally, the methyl groups are
specifically attached to Carbons # 1 & 2 (regardless of numbering clockwise or
counterclockwise). Assembling the name: 1,2-Dimethyl
+ cyclohexane = 1,2-Dimethylcyclohexane. There is no
stereochemistry indicated in this drawing, but the compound can have differences
in the relationship of the two methyl groups to each other.
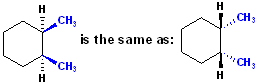 Now,
the stereochemistry for the methyl groups is specific: they are on the same side
of the six membered ring of the cyclohexane. Using the plane of paper as the
reference plane, consider all the Carbon atoms of the cyclohexane to be in that
plane, the methyl groups can either come "out towards us" or "away from us." Of
course, this situation now adds another level to the nomenclature-how is the
stereochemistry indicated in the name? To indicate groups on the same side of
the ring, the prefix cis is used. Now the name is specific, & includes
stereochemistry: cis-1,2-Dimethylcyclohexane.
Now,
the stereochemistry for the methyl groups is specific: they are on the same side
of the six membered ring of the cyclohexane. Using the plane of paper as the
reference plane, consider all the Carbon atoms of the cyclohexane to be in that
plane, the methyl groups can either come "out towards us" or "away from us." Of
course, this situation now adds another level to the nomenclature-how is the
stereochemistry indicated in the name? To indicate groups on the same side of
the ring, the prefix cis is used. Now the name is specific, & includes
stereochemistry: cis-1,2-Dimethylcyclohexane.
 This
structure is also 1,2-Dimethylcyclohexane. A close inspection of the drawing
reveals the difference between this compound and the isomer above: the two
methyl groups are now on opposite sides of the plane (defined by the 6 C atoms
of cyclohexane). The prefix trans (latin for across) is used to describe
this stereochemical relationship. The full name for this structure, including
the stereochemistry is: trans-1,2-Dimethylcyclohexane.
This
structure is also 1,2-Dimethylcyclohexane. A close inspection of the drawing
reveals the difference between this compound and the isomer above: the two
methyl groups are now on opposite sides of the plane (defined by the 6 C atoms
of cyclohexane). The prefix trans (latin for across) is used to describe
this stereochemical relationship. The full name for this structure, including
the stereochemistry is: trans-1,2-Dimethylcyclohexane.
Using the "flat" drawings for cyclic Systems
There is another way of depicting ring systems. In these drawings, the plane of ring is oriented such that the viewer is looking "end on" from the plane:
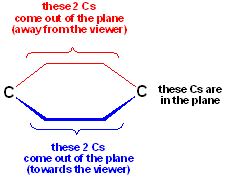
Using this type of drawing, the plane of the paper contains only the 2 Cs indicated in black; there are 2 Cs, which "come out of the plane" towards the viewer (in blue) and they are wedged lines; the remaining two Cs are oriented away from the viewer, "behind the plane" of the paper. However, these positions are NOT wedged-they are simply understood to project away from the viewer. This type of drawing is common with sugar molecules.
Placement of the substitutents in these drawings is most unusual-the remaining bonds are drawn "ceiling to floor" straight up and down:
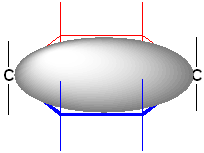
In this drawing, the plane of reference is shown in as a grey oval. Notice the substitutent positions pointing up towards the ceiling and down towards the floor. By using this type of drawing, it is very easy to identify cis & trans isomers. The cis isomers will have the groups on the same side of the "oval" and the trans will be on opposite sides of the oval.
These are all correct drawings of trans-1,2-Dimethylcyclohexane:
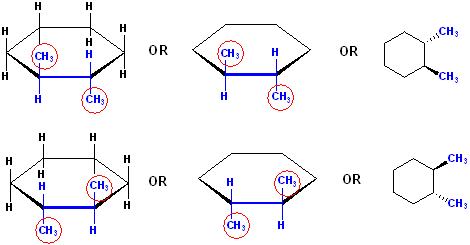 The
drawings on the left, show all atom placements, but notice the relationship of
the methyl groups. They are on opposite sides of the reference plane. It does
not matter which one is 'up" or "down" so long as they are on opposite side of
the reference plane, they will be trans to each other.
The
drawings on the left, show all atom placements, but notice the relationship of
the methyl groups. They are on opposite sides of the reference plane. It does
not matter which one is 'up" or "down" so long as they are on opposite side of
the reference plane, they will be trans to each other.
The drawings in the middle have been simplified. Not all Hs are shown, but are understood to be there. Hydrogen is the ONLY atoms which is handled in this fashion: if not explicitly drawn, the substitutent is assumed to be H atoms. In these structures, only the relevant positions of stereochemistry are shown.
The drawings on the right side are the conventional structures, with the 6 C atoms, in the plan of the paper.
This would be one representation of the cis isomer:
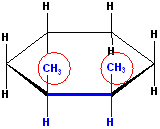
EXERCISE: Draw out the other representations of cis-1,2-Dimethylcyclohexane.
(Click here for answer)
In a similar fashion, cyclopentane can be drawn in a "flat" type of representation:
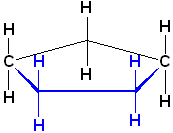 Notice
the substitutent positions pointing up towards the ceiling and down towards the
floor. The bonds in blue are coming out of plane, towards the viewer; once
again, it is understood that two of the carbons are in the plane (indicated by
their symbols: C in the drawing) and the 5th C is orientated away from the
viewer.
Notice
the substitutent positions pointing up towards the ceiling and down towards the
floor. The bonds in blue are coming out of plane, towards the viewer; once
again, it is understood that two of the carbons are in the plane (indicated by
their symbols: C in the drawing) and the 5th C is orientated away from the
viewer.
By using this type of drawing, it is very easy to identify cis & trans isomers.
Consider this cylcopentane:
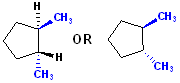 It is
analogous to the cyclohexane above: the 2 methyl groups are on positions 1 & 2
in the cyclopentane ring, so the name becomes:
1,2-Dimethyl + cyclopentane = 1,2-Dimethylcyclopentane.
However, that name alone, does not describe the stereochemistry of the two
methyl groups. If no stereochemistry is indicated in a drawing, then
1,2-Dimethylcyclopentane is an acceptable name. In this drawing, however, the
methyl groups are on opposite sides of the plane (defined by the 5 C atoms of
cyclopentane) and therefore, it is: trans-1,2-Dimethylcyclopentane.
It is
analogous to the cyclohexane above: the 2 methyl groups are on positions 1 & 2
in the cyclopentane ring, so the name becomes:
1,2-Dimethyl + cyclopentane = 1,2-Dimethylcyclopentane.
However, that name alone, does not describe the stereochemistry of the two
methyl groups. If no stereochemistry is indicated in a drawing, then
1,2-Dimethylcyclopentane is an acceptable name. In this drawing, however, the
methyl groups are on opposite sides of the plane (defined by the 5 C atoms of
cyclopentane) and therefore, it is: trans-1,2-Dimethylcyclopentane.
Here are two more representations of trans-1,2-Dimethylcyclopentane:
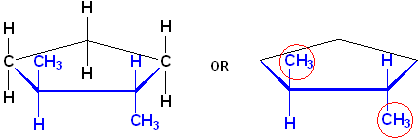
The name of this compound, including the stereochemistry is:
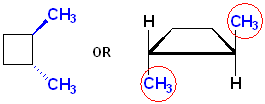
![]()
The name of this compound, including the stereochemistry is:
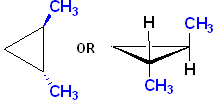
![]()
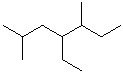
EXERCISE: Write the IUPAC name for each of the following structures.
| Structure | Name | Structure | Name | ||
| 1 | 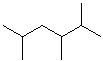 |
|
11 | 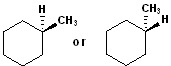 (same structure) |
|
|
2 |
 |
|
12 | 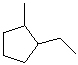 |
|
| 3 | 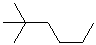 |
|
13 | 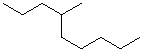 |
|
| 4 | 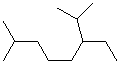 |
|
14 | 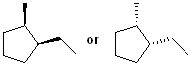 (same structure) |
|
| 5 | 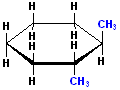 |
|
15 | 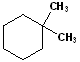 |
|
| 6 | 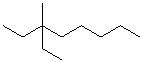 |
|
16 | 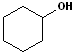 |
|
| 7 | 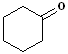 |
|
17 | 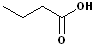 |
|
| 8 | 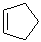 |
|
18 | 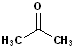 |
|
| 9 | 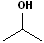 |
|
19 |  |
|
| 10 | 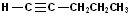 |
|
20 |  |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret