
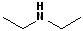
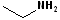
Amines
(The classes of compounds, which may be considered as derivatives of ammonia, in which the H atoms are replaced with alkyl or aryl groups)
Basic IUPAC nomenclature-Amines (See the home page for parent root names, prefixes & suffixes.)
Aliphatic Amines-those in which the N is bonded only to alkyl groups.
1) The longest continuous chain, containing the -N atom will be the parent chain.
2) Number the parent chain, such that the carbon bearing the -N atom has the lowest number.
3) Replace the -e of the alkane by -amine.
The first few members of this family would be named: methane ---> methanamine; ethane---> ethanamine; propane--->propanamine and butane---> butanamine.
Classification of Amines
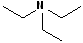
In addition, it is common practice to classify amines according to how many alkyl groups are attached to the N atom. Classifying functional groups in this fashion is especially helpful when studying the variety of reactions of a particular group. When the N is attached to one alkyl group, it is primary (1o); two alkyl groups, secondary (2o); and three alkyl groups, tertiary (3o). Some examples are shown in the table:
 |
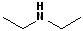 |
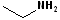 |
 |
|
| IUPAC | 1-butanamine | Diethyl amine | Ethanamine | Triethyl amine |
| Common | n-butyl amine | Diethylamine | Ethylamine | Triethylamine |
| Classification | Primary (1o) | Secondary (2o) | Primary (1o) | Tertiary (3o) |
Common Names
Many amines will also have a common name, which is often used interchangeably with the IUPAC name. The common name is formatted by identifying the alkyl group attached to the N atom and adding, the term amine. Methanamine is also known as methylamine; ethanamine is ethylamine, etc.
Aromatic Amines-those in which the N is bonded to at least one aromatic (aryl) group.
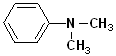
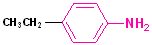
The simplest member of this family is aminobenzene, which is more commonly known as aniline. Other alkyl groups may also be attached to the N atom, and these are indicated by naming the alkyl group and placing a N- as a prefix. This notation (N-) indicates that the groups are attached to the nitrogen atom, as opposed to another site in the structure. Since aromatic amines are derivatives of aniline, this term is retained in the IUPAC nomenclature. (See section on Aromatic Nomenclature)
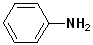 |
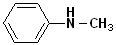 |
 |
 |
 |
| Aniline | N-Methylaniline | N,N-Dimethylaniline | 4-Ethylaniline | 4-Nitroaniline |
| Aminobenzene | N-Methylaminobenzene | N,N-Dimethylaminobenzene | p-Ethylaniline | p-Nitroaniline |
Unsaturated Amines
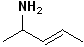
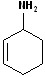
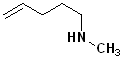
1) For those compounds which contain both a double bond and an amino group, the infix -an- is changed to -en- and the suffix -amine is added.
2) Number the carbon chain, containing both the -N and the alkene to give the amine the lowest carbon number. If there is stereochemistry about the alkene, it too is incorporated. Some simple examples are shown in the Table below:
 |
 |
 |
 |
| (E)-pent-3-en-2-amine | cyclohex-2-enamine | N-methylpent-4-en-1-amine | pent-4-en-1-amine |
EXERCISE: Write the IUPAC name for each of the following structures.
| Structure | Name | Structure | Name | ||
| 1 | 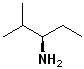 |
|
6 | 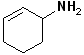 |
|
| 2 | 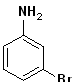 |
|
7 | 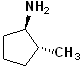 |
|
| 3 | 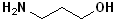 |
|
8 | 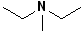 |
|
| 4 | 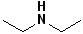 |
|
9 |  |
|
| 5 | 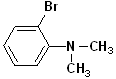 |
|
10 | 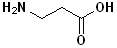 |
|
END OF SECTION
email questions & comments to: Dr. JA Colapret